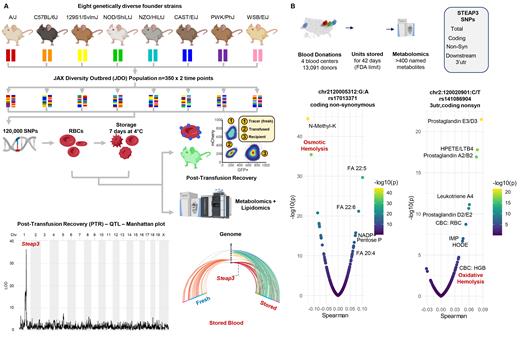
Red blood cell (RBC) storage in the blood bank induces a series of biochemical and morphological alterations, collectively denoted as the storage lesion(s). Membrane damage by lipid peroxidation is a hallmark of the storage lesion, a process thought to be triggered by oxidant stress. To identify the genetics underlying this phenomenon, we leveraged a novel murine system, the Jackson lab Diversity Outbred (JDO) mouse colony, which was obtained by extensively cross-breeding 8 founder mouse strains with extreme genetically heterogeneity ( Figure 1.A). A total of 350 fresh and stored (up to 7 days) murine RBC samples were tested by mass spectrometry-based metabolomics and lipidomics. At the end of storage, post-transfusion recovery (PTR), which measures intra- and extravascular hemolysis and is a gold standard for determining RBC storage quality, was determined by quantifying the percentage of stored RBCs that were still circulating 24h after their transfusion into Ubi-GFP+ recipient mice ( Figure 1.A). Genome wide-association studies identified a strong association between PTR and a polymorphic region on chromosome 1 ( Figure 1.A), which encodes STEAP3, a ferrireductase. This region was associated with altered metabolism of stored RBCs ( Figure 1.A), especially an elevation of lipid peroxidation products (hive plot in Figure 1.A). Mechanistically, these data support a role for STEAP3 in promoting Fenton and Haber-Weiss chemistry in iron-loaded RBCs. Specifically, ferrous iron participates in this chemistry, becoming oxidized to its ferric state with concomitant generation of hydroxyl or hydroperoxyl radicals; these in turn attack fatty acids (preferentially, poly- and highly-unsaturated fatty acids, such as octadecadienoic and eicosatetraenoic acid), thereby promoting lipid peroxidation (to hydroxyoctadecadienoic (HODE) and hydroxyeicosatetraenoic (HETE) acids, respectively). By reducing Fe 3+ to Fe 2+, STEAP3 can shift the balance of this reaction by increasing the availability of the reactant, thereby favoring the generation of product radicals; this process is analogous to ferroptosis, as described in other biological systems.
To extend the relevance of these findings to humans, we performed metabolomics on end-of-storage (i.e., Day 42) packed RBCs from 13,091 human donors in the Recipient Epidemiology and Donor Evaluation Study (REDS RBC Omics; Figure 1.B), enrolled in four blood centers across the United States. We stratified donors based on oxylipin levels (e.g., acids - HETEs - Figure 1.B) and common STEAP3 polymorphisms (e.g., a representative non-synonymous coding single nucleotide polymorphism (SNP), rs17013371, was observed with ~10% prevalence in these donors), along with 37 other common SNPs observed in this study ( Figure 1.B). When correlating metabolic and hematological parameters to common STEAP3 non-synonymous coding SNPs, we found strong associations of such polymorphisms with RBC susceptibility to hemolysis (either osmotic or oxidative), lipid peroxidation, RBC numbers, and hemoglobin levels ( Figure 1.B). These associations held true for a subset of the original donors with extreme hemolytic propensity (n=643) who donated a second RBC unit that was again stored for 42 (i.e., the “recalled donor population”). In the initial (n=13,091) and recalled donor (n=643) populations, lipid peroxidation products, HETEs, and HODEs ranked as the top predictors of hemolysis and vesiculation.
In summary, we identified a role for the STEAP3 ferrireductase in contributing to a ferroptosis-like phenotype of lipid peroxidation that is associated with increased susceptibility to hemolysis in vitro and in vivo in murine and human RBCs following storage under blood bank conditions.
Figure 1 - A. Jackson Lab diversity outbred (JDO) mice were bred from 8 founder mouse strains with extreme genetic heterogeneity. A total of 350 fresh and stored murine red blood cells (RBCs) were tested for metabolomics, lipidomics, and post-transfusion recovery (PTR). A polymorphic region on chromosome 1, coding for the ferrireductase STEAP3 was associated with heterogeneous PTR and elevation in lipid peroxidation products. B. Metabolomics analyses of 13,091 donors from the REDS RBC Omics identified an association between the end of storage levels of oxylipins and common polymorphisms for STEAP3, both linked to an increased RBC susceptibility to hemolysis (either osmotic or oxidative.
Disclosures
D'Alessandro:Omix Technologies Inc: Current equity holder in private company; Macopharma: Consultancy; Hemanext Inc: Consultancy. Nemkov:Omix Technologies Inc: Current equity holder in private company. Zimring:Rubius Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal